What is Aluminium Hydroxide?
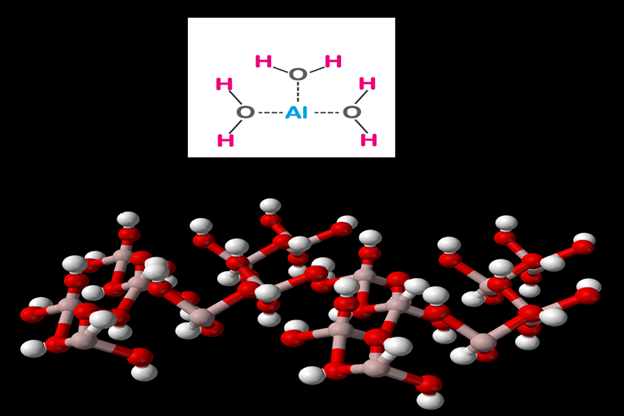
Aluminium hydroxide is a chemical compound that contains one aluminium atom and three hydroxyl (OH) groups. Aluminium hydroxide is found as a white powder and in nature as a bauxite mine.
Aluminium hydroxide has properties such as heat resistance, anti-acidity, waterproof and flame retardant properties. Due to these properties, it is used in many industries and applications. For example, aluminium hydroxide is used as a fire retardant in construction and consumer applications. Due to its ability to absorb heat and water, this material can effectively control heat and help in delaying and preventing the spread of fire in the environment.
Aluminium hydroxide is also used in the construction industry, glass industry, plastic production, chemicals, rubber industry, electronics industry, etc. as a filler, waterproof material, catalyst, antioxidant agent, and in the paper cellulose industry.
The use of aluminium hydroxide in the mentioned cases requires compliance with safety principles and relevant instructions. Also, for the optimal use of its properties, attention should be paid to the proper fit with the environment and the needs used.
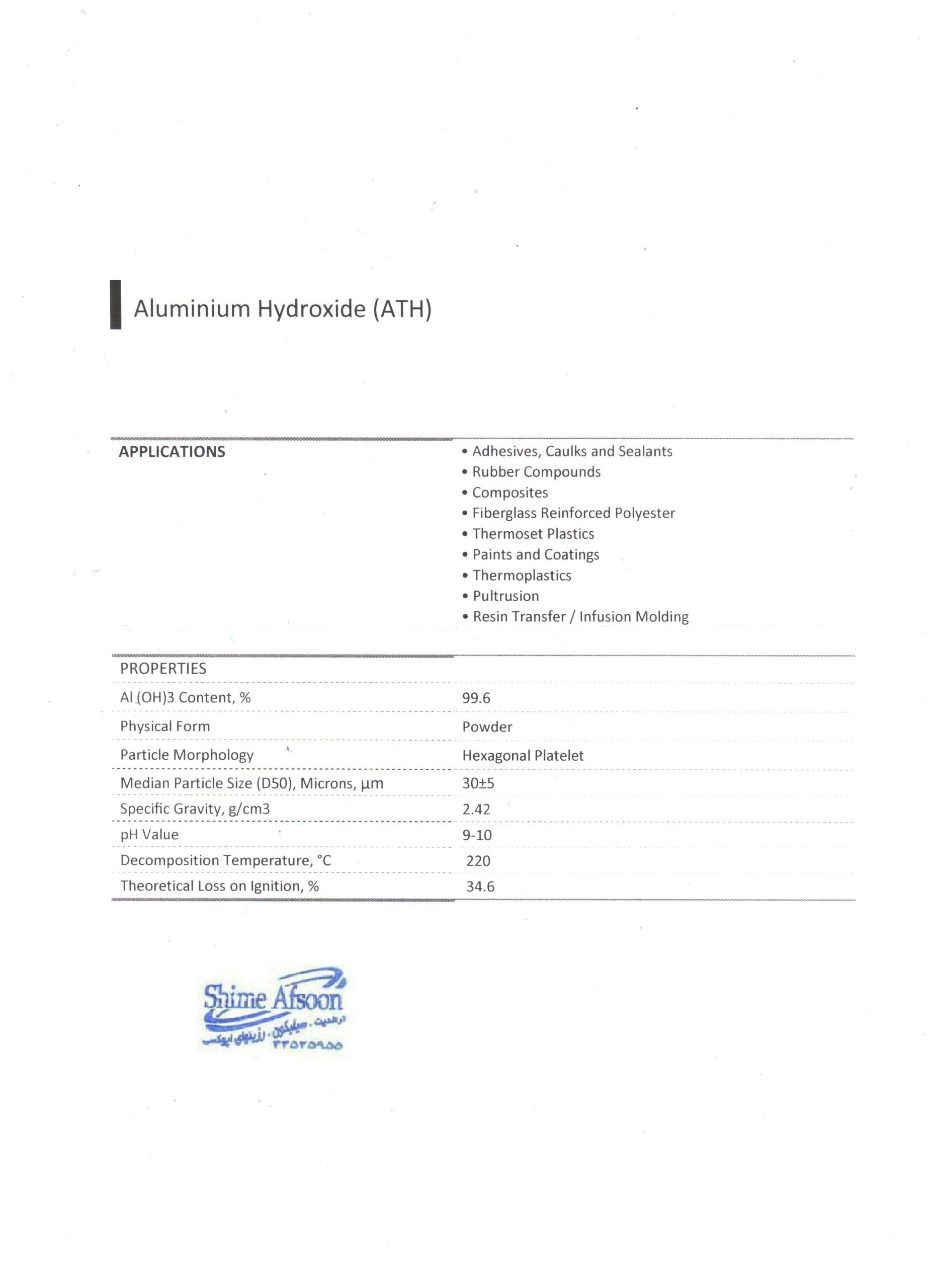
Properties of Aluminium Hydroxide (fire retardant ATH)
Pure aluminium hydroxide has the form of a white bulk powder or granule with a density of about 2.42 g/ml. Aluminium hydroxide does not dissolve in water, but only in bases and acids. You can expect aluminium hydroxide to act as an amphoteric agent in water.
If a strong base is present, aluminium hydroxide acts as an acid. And if a strong acid is present, it acts as a strong base.
Aluminium hydroxide should be handled with caution as exposure can cause irritation. However, there will be only minor and residual injuries. Regarding flammability, aluminium hydroxide is not flammable and does not burn. In addition, aluminium hydroxide is not reactive, therefore, it is stable in both fire and water conditions.
- This substance is a white powder with a particle size of 3 to 100 microns.
- It is odorless and non-flammable.
- It has an alkaline pH and before ripening
- Density (grams per cubic centimeter): 2.42
- It is destroyed before reaching the melting point.
- It is insoluble in water and soluble in acid.
- Molecular weight: 78 g/mol
- It is stable up to 180 degrees Celsius.
The price of Aluminium Hydroxide
As one of the suppliers of chemical products in the country, Shimi Afsoon provides a wide range of chemicals from reputable brands to manufacturers. For more information about the price of aluminium hydroxide, you can contact our colleagues. For information on how to buy aluminium hydroxide, contact our experts at Shimi Afsoon.

The best price of Aluminium Hydroxide from Shimi Afsoon
According to field studies, our country has more than 20 million tons of extractable bauxite soil, which is mainly located in the northeast. Other than that, some researches have been done to explore mines in the center and southwest of the country. The annual production of aluminium hydroxide in the world is about 100 million tons per year, of which more than 90% is used for the production of aluminium oxide.
An important part of this aluminium oxide production is also used for the extraction of aluminium metal. 10% of aluminium hydroxide is also used for chemical and fireproof applications.
Shimi Afsoon (aluminium hydroxide producer) is proud to meet an important part of the needs of the chemical industries by providing high purity aluminium hydroxide powder in a completely dry and fine-grained form.
Application of Aluminium Hydroxide
Aluminium hydroxide has many uses. Some people believe that these uses are truly endless. Just to show the breadth of applications, aluminium hydroxide is used as a colorant, water purifier, cosmetic ingredient, and even as an element for photographic processes.
There are also applications of minor features in ceramics and construction. But the most important field in which aluminium hydroxide is used is medicine.
Aluminium hydroxide is used for the production of aluminium metal and also for the production of many aluminium-containing compounds such as sodium aluminate, aluminium sulphate, aluminium chloride, etc. It is used in making glass, ceramics, refractory products, fire extinguishers, water treatment and abrasive materials.
Aluminium hydroxide is used as a filler in polymers such as polyester resin and epoxy resin, plastics and sponge rubber. It is also used in the pharmaceutical industry as an anti-stomach acid drug.
Aluminium hydroxide and similar compounds have wide applications in industry, health and pharmaceuticals. In the field of water treatment, aluminium sulphate Al2(SO4)3 or alum (usually potassium aluminium sulphate KAl(SO4)2) is mixed with calcium hydroxide Ca(OH)2 in a water tank in order to purify water. The reaction of these compounds with each other causes the appearance of aluminium gelatinous deposits.
When sediments settle, suspended particles, pollutants and bacteria in polluted water are absorbed by its compounds. Aluminium hydroxide precipitates are later separated from water by special filters. The ability of aluminium hydroxide to absorb elements is indicative of its other uses in some chemical processes, for example, its use as a filter, ion displacement, and in chromatography.

Application in pharmaceuticals
Considering that aluminium hydroxide is able to neutralize acids, it acts as a natural antacid. Aluminium hydroxide also has a very useful property because it stimulates the immune system of the human body. In addition, various vaccines, including those used to treat hepatitis B, hepatitis A, and tetanus, are prepared using aluminium hydroxide.
It can also be used to treat kidney patients who have high levels of phosphate in the blood due to kidney failure. This beneficial property is due to the ability of aluminium hydroxide to bind with phosphates. After binding with aluminium hydroxide, phosphates are easily removed from the human body.
Application in paint industry
Aluminium hydroxide is also used as a color stabilizer. In most cases, compounds are deposited on the fiber that is placed to be dyed. The material is then floated in the dye pool. The color of the final product will also depend on the combination of dyes and stabilizers used in the process.
A similar process is used in the production of paint pigments. The applied color along with the precipitated aluminium hydroxide and the insoluble compounds formed are then filtered.
Application of aluminium hydroxide in health products
There are various uses for aluminium hydroxide in the field of cosmetics. Aluminium hydroxide is mostly used to make lipsticks, cosmetics and other skin care products. It is used there because it is quite stable and non-toxic to humans.
Sometimes cosmetic manufacturers use aluminium hydroxide to make skin cleansers, sunscreen products, body lotions, and moisturizers.
Personal care products, for example, shampoo, toothpaste, deodorant and many others also contain the use of aluminium hydroxide. Sometimes aluminium hydroxide is also used to protect human skin.

Industrial application
Concrete cannot be produced without aluminium hydroxide. In the concrete production stage, aluminium hydroxide is added to cement. It is also very useful because cement with the addition of aluminium hydroxide dries quickly when exposed to heat. Ceramics and glass for industrial and domestic use are produced using aluminium hydroxide.
The most useful property of aluminium hydroxide when added to glass is that it makes the glass heat resistant. This is possible because, as mentioned earlier, aluminium hydroxide is not flammable and has a high melting point. It appears that aluminium hydroxide combined with polymers is a very good fire retardant.
Do not forget that aluminium hydroxide does not dissolve in water. For this reason, it can be used by adding it to textiles to produce waterproof clothing. In addition, aluminium hydroxide will also be very useful when there is a need to adhere natural dyes to fabric. In this case, aluminium hydroxide is used as a drying agent.
In cases where the fabrics are resistant to color, any color is used. In such a situation, a pigment material allows the fabric to be permeated by the dye. Another example of the use of aluminium hydroxide is when it is used to make some paints resistant to fire.
Other fields of application
Considering how aluminium hydroxide is actively used in various fields, we cannot exclude other fields of its application. Apart from what we mentioned above, aluminium hydroxide as well as any other aluminium compound is used for water purification in order to remove particles and all kinds of impurities.
In the manufacture of inks, aluminium hydroxide acts as a stabilizer and preservative. Aluminium hydroxide can also be used as chromatography in laboratories to separate chemicals into different compounds.
Aluminium hydroxide medicine
Aluminium hydroxide is a drug used in the management and treatment of acid indigestion. This drug is in the group of antacid drugs.
Aluminium hydroxide sales are often prescribed orally for temporary relief of heartburn or gastroesophageal reflux. It may be used topically, temporarily, to protect and soothe scratched and abraded skin, minor wounds and burns, and skin irritations caused by friction and rubbing. Patients may also receive it as a mouthwash to treat chemotherapy-induced oral mucositis.
In addition, due to its ability to increase phagocytosis and stimulate immune responses, it is approved for use as an adjuvant in numerous vaccines. It acts as a phosphate binder in patients with chronic kidney disease. However, its use in this way is rare due to the risk of side effects. Typically, oral aluminium hydroxide is a liquid formulation of both aluminium and magnesium hydroxide.
Mechanism of action
Aluminium hydroxide al(oh)3 is decomposed into al3+ and oh- in the stomach. Then the released hydroxide groups are attached to free protons and finally produce water and insoluble aluminium salts, mainly Al(Cl)3 in the stomach. Proton binding increases the overall pH of the stomach, which means it is less acidic and reduces the symptoms of indigestion. The aluminium salt produced is mainly excreted in the feces, and less than 1% of the bioavailable aluminium is absorbed in the gastrointestinal tract.
Aluminium exists in a fixed state in the body, so the body manages a transient increase in aluminium absorption from use as an antacid with an equivalent increase in urinary excretion of unmodified aluminium. However, the set point of that steady state may increase with long-term aluminium intake, so aluminium hydroxide therapy should not be considered as a long-term solution for patients with acid indigestion.
In addition, patients with chronic kidney disease may not be able to excrete increased aluminium and therefore should be carefully considered. Topical aluminium hydroxide creates an acidic, hydrophilic monolayer over the irritation area that acts as a protective barrier to prevent infection and prevent bacterial growth within the wound.
Management
Aluminium hydroxide when used as an antacid must be delivered orally. Shake the aluminium hydroxide suspension well before use. It should be taken 5 to 6 times a day after meals and before going to bed, not exceeding 3.84 grams per 24 hours. The patient should follow the dose with water.
Aluminium hydroxide production method
In aluminium sulphate solution, alkaline solution is added to the stirrer and then the sediments are washed, filtered and dried at low temperature. In the preparation process, solution concentration, temperature, reaction temperature control and dry temperature affect the quality of the products.
Aluminium sulphate and aluminium ash were used to produce aluminium sulphate and then decomposed with ammonium bicarbonate to produce aluminium hydroxide. Or sodium aluminate solution and aluminium sulphate solution are neutralized to pH 6.5 and aluminium hydroxide is precipitated. After washing and pressure filtration, it is dried at 70 to 80 degrees Celsius and then crushed to produce aluminium hydroxide. Its reaction formula:
Hydrogen Peroxide
Under the conditions of 40-60°C, pH>12, the sodium aluminate irrigation solution is injected into carbon dioxide, and the sodium aluminate irrigation solution is not added at normal temperature and is not added at normal temperature.
Al (OC2H5) 3 and 1% ~ 20% ethanol amine NH2C2H4OH are synchronized and hydrolyzed at 20 ~ 60 °C and the gel is treated for several months.
(III) Hydroxide:
Blowing dioxide in aqueous sodium aluminate solution at room temperature.
Aluminium amalgam was hydrolysed at normal temperature.
Al (OC2H5) 3 is hydrolysed below.
Method of neutralizing aluminium chloride solution with alkaline hydroxide or ammonia.
A bauxite water is oxidized by hydrothermal treatment of aluminium hydroxide at 150 to 300 °C in aluminium hydroxide solution or alloy irrigation solution and with aluminium ammonia with boiling water. The stable area of bauxite is 275 to 425 degrees and the vapor pressure is above 140 amps (1tm = 98.066kPa). It has been reported that bauxite is obtained using aluminium hydroxide hydrothermal method and alumina hydrothermal treatment and alumina oxide produced by bauxite chemical vacuum.
In summary; The production of aluminium hydroxide and alumina is based on the Bayer process. In this process, bauxite is first dissolved in caustic soda. Using sedimentation and filtration, the dissolution residues are separated from each other. Aluminum hydroxide is filtered and then calcined at a temperature of about 1000 degrees Celsius. Calcination process is done to remove excess water.
The product obtained from this process is white alumina powder. Bayer process requires a lot of energy in general and many efforts have been made in this field.
Aluminium hydroxide in vaccines
Aluminium hydroxide is the most common chemical used as an adjuvant in vaccines. Despite its widespread use, surprisingly, the mechanism of action of aluminium hydroxide-based adjuvants is still not fully understood. Current explanations for the mode of action of aluminium hydroxide-based adjuvants include a reservoir effect, a phagocytic effect, and activation of the proinflammatory NLRP3 pathway.
Together, these galvanize innate and adaptive immune responses and activate the complement system. Factors that have a profound effect on the responses elicited by aluminium hydroxide-based adjuvant applications are adsorption speed, adsorption strength, aluminium hydroxide particle size and uniformity, adjuvant dosage, and the nature of antigens. Although vaccines containing aluminium hydroxide-based adjuvants are useful, they sometimes cause adverse reactions.
In addition, these vaccines cannot be stored frozen. Until recently, ATH-based adjuvants were known to preferentially induce Th2-type immune responses. However, the results of recent studies show that depending on the route of vaccination, aluminium hydroxide-based adjuvants can increase Th1 and Th2 cell responses. Advances in systems biology have opened new avenues to study aluminium hydroxide-based adjuvant mechanisms.
These help scale new frontiers in ATH-based adjuvant research, including formulation improvement, the use of aluminium hydroxide nanoparticles, and the development of composite adjuvants.
As an adjuvant in vaccines, aluminium hydroxide is known to increase macrophage phagocytosis, possibly through upregulation of nlrp3-inflammasome and increased uptake of target antigen. In addition, it facilitates what is known as the reservoir effect, whereby antigens accumulate on and around the molecule, which helps prevent their degradation in the body.

Effects of Aluminium Hydroxide
Common side effects or health problems may include:
- Nausea
- Vomit
- Back acidity
- Aluminium poisoning
- Low blood phosphate (hypophosphatemia)
- Chalky taste
- Constipation (this can lead to haemorrhoids or a bowel obstruction)
- Stool retention
- Stomach cramps
- Alkaline milk syndrome
- Softening of the bones
* Serious side effects of aluminium hydroxide include:
- Black/tarry stools
- Mental/behavioural changes (e.g., confusion, deep sleep)
- Pain with urination
- Stomach/abdominal pain
- Vomiting that looks like coffee grounds.
These materials do not contain all possible side effects and others may occur. You should consult your doctor for more information about side effects.
Poisoning, seizures, bone softening and encephalopathy are among the important effects that have been recorded in the use of this substance. Before administering aluminium hydroxide, a test should be taken from the patients that they do not have problems in consuming this substance, because these results have a lot to do with the use of ATH as a phosphate binder in patients undergoing dialysis. Most of the reports of encephalopathy and osteomalacia date from a period when water purification standards for dialysis were not as strong as they are now, possibly confounding the association of oral aluminium with toxicity.
Reversal of aluminium hydroxide toxicity occurs when the drug is discontinued. Aluminium hydroxide adjuvanted vaccines have shown no toxic effects, primarily due to the low concentration associated with both applications. Seizures, osteomalacia, and encephalopathy are well-documented toxic effects of aluminium hydroxide. Before administering aluminium hydroxide, patients should be asked about any renal problems, as these results are strongly associated with the use of aluminium hydroxides as a phosphate binder in dialysis patients.
are not as potent as they are now, possibly confounding the relationship of oral aluminium to toxicity. Reversal of aluminium hydroxide toxicity occurs with discontinuation of the drug. Topical ATH and aluminium hydroxide adjuvant vaccines do not produce any toxic effects, primarily due to the low concentration associated with both applications.
Aluminium Hydroxide ATH packaging
25 kg